Moderna Vaccine Update Phase 3

The vaccine known as mrna 1273 was co developed by the cambridge massachusetts based biotechnology company moderna inc and the national institute of allergy and infectious diseases niaid part of the national institutes of health.
Moderna vaccine update phase 3. Covid vaccine candidate from cambridge based company was nearly 95 effective during its phase 3 human trials updated nov 16 2020. 25 654 participants have received their second vaccination. Moderna s covid 19 vaccine candidate shows 94 5 efficacy in phase 3 trials november 16 2020 11 30 am moderna announced monday that their covid 19 vaccine candidate is showing 94 5 efficacy. Posted nov 16 2020 facebook share.
They have yet to bring one to the market. A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun. Moderna s commitment to diversity inclusion. The late stage trial will include 30 000 participants and is expected.
O n june 11 biotech company moderna announced it had finalized plans for phase 3 testing of its covid 19 vaccine candidate. Kdka s amy wadas reports. View the phase 3 cove study protocol. Moderna is committed to advancing the clinical development of mrna 1273 as safely and quickly as possible to demonstrate our vaccine s ability to significantly reduce the risk of covid 19 disease vaccine candidate mrna 127 is currently in a phase ii clinical trial which will enrol 600 healthy participants aged 18 and above.
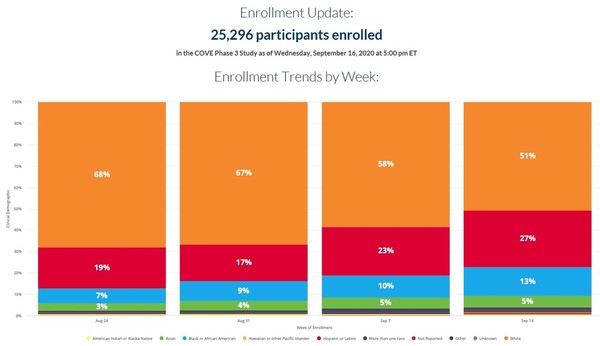
Moderna is trailing pfizer and biontech nasdaq bntx which had enrolled 25 189 patients in the phase 2 3 clinical trial testing its coronavirus vaccine bnt162b2 as of an update on sep. In the cove phase 3 study as of thursday october 22 2020. Phase 3 moderna develops vaccines based on messenger rna mrna to produce viral proteins in the body. In january they began developing a vaccine for the.