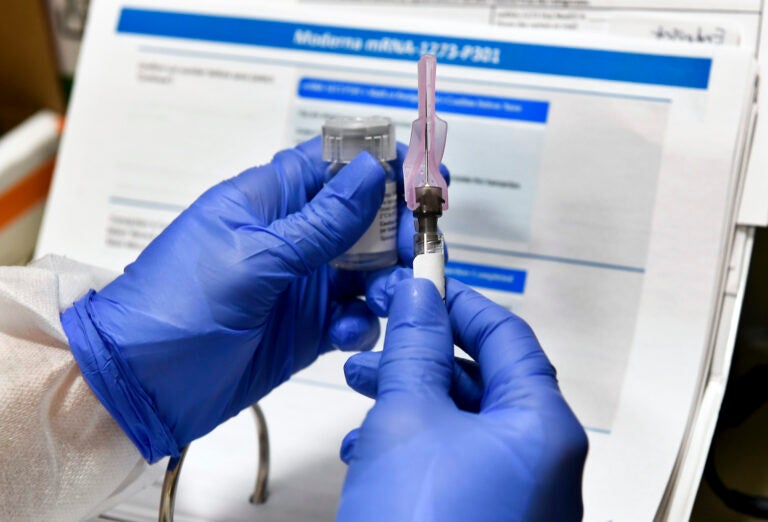
Moderna Vaccine Two Doses
Uk now has access to seven million shots of two dose vaccine enough to inoculate 3 5 million britons.
Moderna vaccine two doses. Two doses of moderna s coronavirus vaccine protected mice from infection for 13 weeks and a single shot blocked the virus for 7 weeks. Could have as many as 60 million doses of vaccine available by the end of the year reuters reported. By the end of the year moderna expects to have approximately 20 million doses of the vaccine ready to ship in the u s the company said. It s the second.
Moderna s vaccine was an important addition to our portfolio and securing an additional two million doses further adds to the protection we can provide to the public to end the pandemic related. Britain has secured two million doses of moderna inc s covid 19 vaccine candidate to be available in europe as early as the spring the government said on sunday in addition to the 5 million. Moderna a massachusetts based biotech firm announced monday that its vaccine is 94 5 effective in preventing covid 19. Researchers still aren t sure if the vaccine will work based on the new evidence but the initial results are promising for a two dose vaccine.
Overall if both the moderna and pfizer vaccines are approved by the fda the u s. Food and drug administration fda with two month. Multiple doses gives the immune system the opportunity to produce a specific and effective. Two coronavirus vaccine candidates now lead the pack.
At that time more than 25 650 participants had received their second vaccination in the two dose schedule. Moderna now must provide the u s. Pfizer biontech s and moderna s vaccines both require two doses which is common for many vaccines. It said it remains on track to manufacture 500 million to.