Moderna Vaccine Phase 4
Biontech and pfizer s vaccine bnt162b2 and moderna s mrna 1273 are both mrna vaccines that require freezing levels of storage in the range of negative 94 degrees.
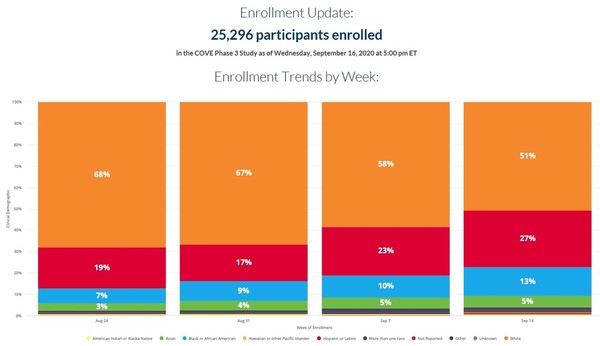
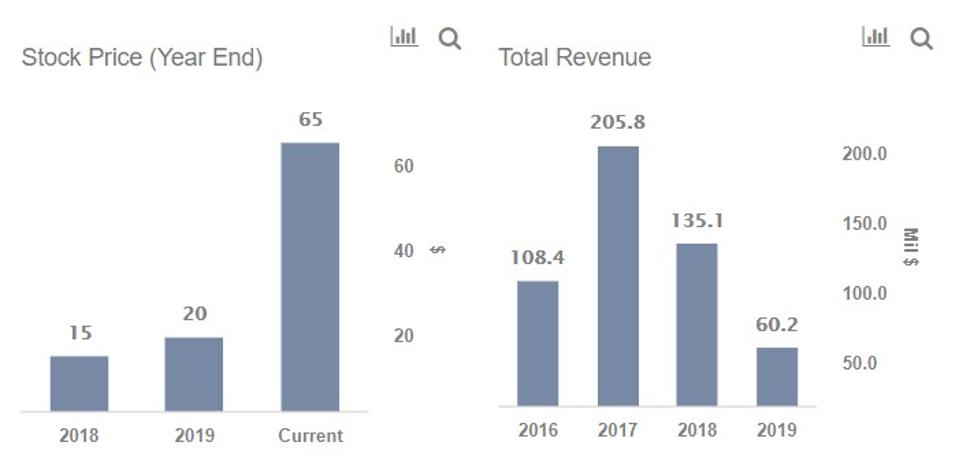
Moderna vaccine phase 4. Moderna s stock fell by as much as 9 4 in midday trading thursday after a report said the biotech company s late stage trial for a potential coronavirus vaccine will be delayed possibly by a few. The vaccine requires two doses a month apart. And at 6 43 a m. There were no serious side effects.
But more than half the study participants reported flu like reactions to. Moderna s covid 19 vaccine is an mrna vaccine that relies on messenger rna to deliver instructions to the cells in your body which then produce small parts of the covid 19 virus that are just. For mrna 1273 we were able to leverage our experience in vaccines to move rapidly on design and manufacture of material for the phase 1 clinical trial. To date moderna has demonstrated positive phase 1 data readouts for 6 prophylactic vaccines h10n8 h7n9 rsv chikungunya virus hmpv piv3 and cmv.
But when administered at a half dose and then a full dose the vaccine can. On july 27 the first volunteer in moderna s 30 000 person. Overall results from phase three of the oxford astrazeneca trial show the vaccine is 70 4 per cent effective on average. Moderna said preliminary phase three trial data shows its coronavirus vaccine is more than 94 effective in preventing covid 19.
And european regulators to allow emergency use of its covid 19 vaccine as new study results confirm the shots offer strong protection. Moderna s vaccine reached phase i human trials on march 16 only 63 days after the company began developing it. The company s ceo stephane bancel called it a game changer. Moderna is expected to launch a phase 2 trial of the vaccine in the coming weeks with phase three expected to occur in july.