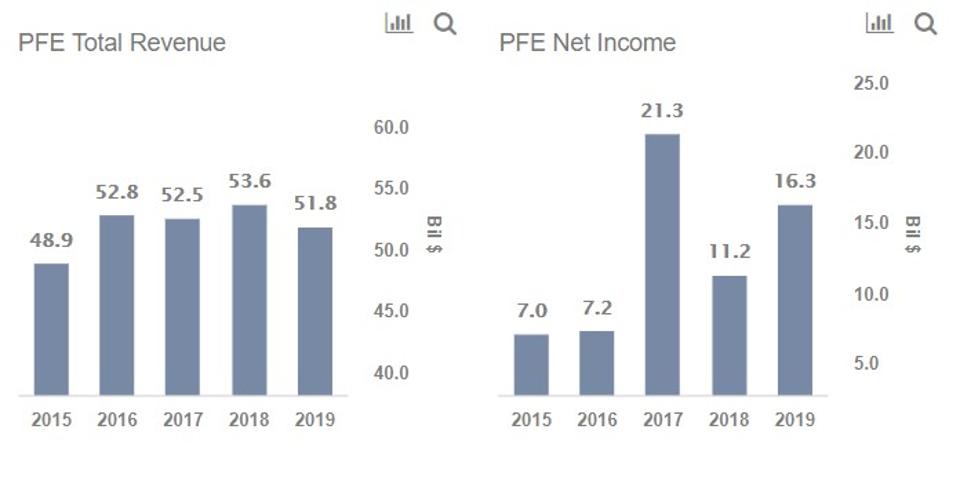
Moderna Vaccine Phase 3

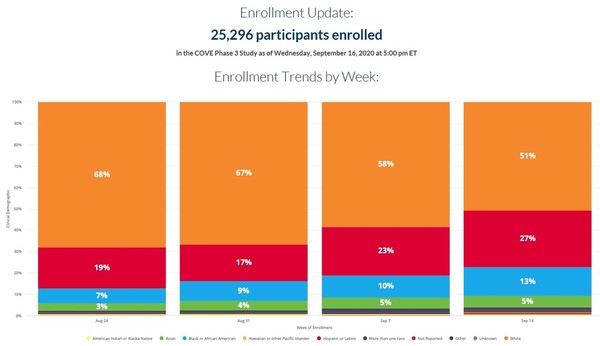
The late stage trial will include 30 000 participants and is expected.
Moderna vaccine phase 3. Moderna s coronavirus vaccine entered a new and crucial phase of testing on monday. The vaccine known as mrna 1273 was co developed by the cambridge massachusetts based biotechnology company moderna inc and the national institute of allergy and infectious diseases niaid part of the national institutes of health. A phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 covid 19 in adults has begun. Moderna have announced that their vaccine candidate against covid 19 has demonstrated 94 5 efficacy in the first interim analysis from the phase 3 vaccine trials.
The company s ceo stephane bancel called it a game changer. Prof adrian hill professor of human genetics director of the jenner institute co director oxford martin programme on vaccines and fellow of magdalen college said. O n june 11 biotech company moderna announced it had finalized plans for phase 3 testing of its covid 19 vaccine candidate. Find out why this vaccine technology is promising but not without its skeptics.
Moderna said preliminary phase three trial data shows its coronavirus vaccine is more than 94 effective in preventing covid 19. The biotech firm s leading candidate has just entered phase three clinical trials in the u s.