Moderna Vaccine On Hold

Auburn based company sio2 materials science is producing unique vials that hold doses of moderna s covid 19 vaccine.
Moderna vaccine on hold. Drugmaker moderna says its covid 19 vaccine is 94 5 percent effective based on preliminary data from its ongoing trial making it the second us company in a week to report results that far exceed. Pfizer if authorized expects to produce up to 50 million vaccine doses in 2020 and 1 3 billion in 2021. If the numbers hold up when the vaccine s released to the general public. Asia pacific stocks may trade higher following a strong.
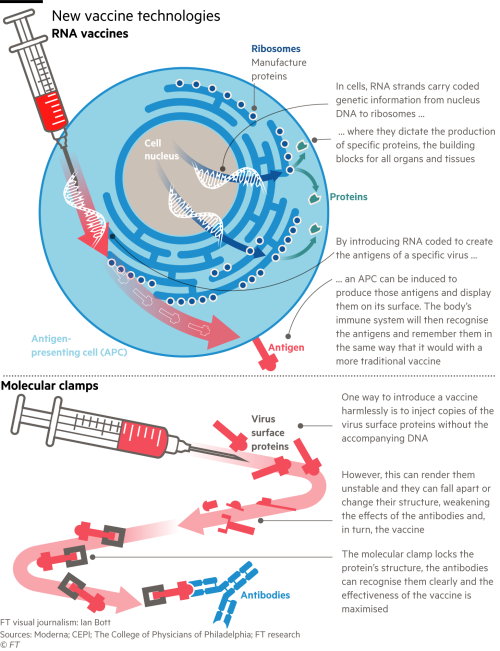
Uic doctors say moderna covid 19 vaccine trial may have information on effectiveness by december by megan hickey october 26 2020 at 5 54 pm filed under. To have a high success rate. Moderna s vaccine is a two part injection with the second dose coming 28 days after the first. Moderna covid vaccine begins phase 3 03 30.
For us we ve been shipping vials to the companies. Cbs 2 investigators chicago. If these full data sets hold when the full data comes out we may have two highly effective vaccines against covid gottlieb a board member of pfizer said on squawk box once we get these. Phase three trial of a coronavirus vaccine has failed to attract a sufficient number of racial minorities according to experts.
The safety board can also put the trial on hold if there is evidence that a participant may have been harmed. Moderna and pfizer plan to apply for emergency authorization from the fda this month.