Moderna Vaccine Mrna 1273

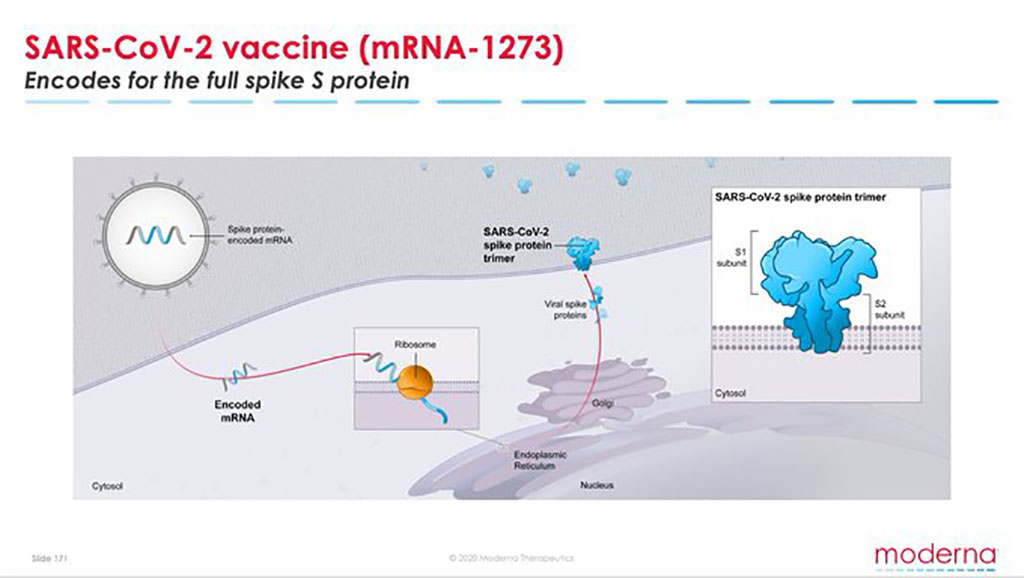
In this case moderna s mrna 1273 is programmed to make your cells produce the coronavirus infamous coronavirus spike protein that gives the virus its crown like appearance corona is.
Moderna vaccine mrna 1273. Phase 1 trial results of the vaccine mrna 1273 have been found promising. Reviewed by judith stewart bpharm last updated on jul 27 2020. Mrna is an information molecule and moderna inc. The investigational vaccine directs the body s cells to express the spike protein to elicit a broad immune response.
Mrna 1273 is an mrna vaccine candidate against the novel coronavirus sars cov 2 encoding for a prefusion stabilized form of the spike s protein which was selected by moderna in collaboration with investigators at the niaid vaccine research center. Mrna 1273 is a novel lipid nanoparticle lnp encapsulated mrna based vaccine that encodes for a full length prefusion stabilized spike s protein of sars cov 2. Moderna covid 19 vaccine coronavirus vaccine latest update today. This clinical trial is designed to assess the safety reactogenicity and immunogenicity of mrna 1273 manufactured by modernatx inc.
100 µg of mrna 1273 vaccine or a placebo control in a 1 1 randomization ratio. Designs its mrna vaccines using the sequence of the virus not. On the surface moderna has a slight lead on efficacy. The spike protein on its surface facilitates entry into a cell.
A look at what the vaccine is composed of and how it works what the trials showed and what are the many stages that remain. Mrna 1273 has a vaccine efficacy of 94 5 based on the 90 cases of covid 19 in patients who received placebo and 5 cases in patients who got. The mrna 1273 vaccine candidate manufactured by moderna encodes the s 2p antigen consisting of the sars cov 2 glycoprotein with a transmembrane anchor and an intact s1 s2 cleavage site. Prevention of covid 19 mrna 1273 is an investigational mrna vaccine against sars cov 2 in development for the prevention of covid 19.
Assignment will be stratified by age and health risk. Sars cov 2 is the virus that causes covid 19. Mrna 1273 fda approval status.